by Štefan Emrich and Niki Popper (dwh GmbH, TU Wien, DEXHELPP)
COVID-19 brought unprecedented publicity for modelling and simulation. But a broad audience was left with very little information about what modern simulation models have to take into account and how valuable they have become as decision-support-tool. And how versatile: by far not limited to health-care.
When information about COVID-19 became available in early 2020, the questions were not if but when the first case would be recorded in Austria – and how best to prepare the healthcare system. The Generic Population Concept (GEPOC) became one of the key tools to answer this question.
The GEPOC is one of the major accomplishments of the research project “Decision Support for Health Policy and Planning” (DEXHELPP). This project was a collaboration between nine partners, amongst them dwh GmbH, the university TU Wien and SBA Research, who provided solutions for secure data processes [L1]. The GEPOC [1], which has been in development since 2014, is a reusable population model that can evolve into a versatile simulation framework to support policymakers in decision-making around population-related research questions.
The research team chose an agent-based (AB) approach for this platform, which makes it possible to model each of the country’s almost 9 million citizens individually. It allows flexible integration of “personal” characteristics and features, such as sex, age, workplace, level of education, preferences (e.g. commute to work by car or public transport) and requirements (e.g. medication, childcare). This framework can virtually imitate the behaviour and contacts of each citizen. For COVID-19 this was just what was needed: a tool that simulate the influence of individual behaviour on the epidemic spread – and hence help evaluate the effect of measures on the number of reported cases.
Data from the Austrian Bureau of Statistics (Statistik Austria) was used in the model to precisely depict Austria’s demographic structure, including predictions of international and national migration and population development. One problem was that available data for residence is only precise to the municipality. Consequently, we did not have an accurate picture of population density, which is strongly influenced by topography and settlement and is highly relevant for many issues, including epidemic spread. To overcome this we included information from the Global Human Settlement Project in the model, and distributed the agents accordingly. Each agent is assigned a residence coordinate, which mitigates problems due to changes of administrative regions over time (changes, splits or mergers of regions). This also allows for different aggregation levels (e.g. lattice grids of a given granularity or aggregation on different levels, such as levels of settlement, district or province).
The social and contact behaviour, especially daily routines (going to workplaces, schools, etc.) was based on the POLYMOD study, a large survey on social contact behaviour that is relevant to many aspects of human interactions (from demand for transportation to the spread of infectious diseases), and information from Statistik Austria on variables such as workplace and school sizes.
GEPOC was designed to cope with extremely different time scales, ranging from a few days or weeks (e.g. short time resource planning) to years or decades (e.g. observing the impact of demographic changes on the healthcare landscape).
This poses yet another challenge: AB-models need a time-update strategy that correctly coordinates and processes all events and interactions. Usually either a time-continuous or a time-discrete concept is used, but neither is optimally suited for a generic population model. Consequently, we developed a new logic combining both strategies.
The great power and versatility of GEPOC lie in its extensions: the ability to add various modules and layers to the model. For example, having the precise geographic and demographic location of the population, incorporating information about railroads, stations and timetables, as well as information about schools and workplaces, allows us to determine where rail services are needed. Information about locations, specialisation and opening hours of doctors, and available healthcare services shows which areas are undersupplied and where different infrastructure might be needed in ten years due to demographic changes.
Modules that simulate diseases can also be incorporated. There are containing rules describing the disease, such as predisposing factors (e.g. sex, age, predisposition, underlying health conditions or medication), patient pathways and the epidemiology of the disease. Of course, disease modules can be coupled with infrastructure layers, e.g. to assess the impact of epidemics on the healthcare system or areas where resources will become critical. Due to the modularity of the tool, it is possible to add more than one disease module to simulate and study the reciprocal effects of concurrent diseases.
It is always challenging for decision-makers to formulate an adequate response to diseases – especially epidemics. Possible actions are added through “policy modules” where implementations of interventions such as immunisation, medication, contact-reduction policies, contact tracing, closures of organisations such as schools and workplaces and quarantine are described.
This framework has been proven and tested in several research projects with such diverse applications as: (i) evaluating Austrian vaccination rates for measles and polio (for the Austrian Ministry of Health’s report to the WHO [L2]); (ii) modelling re-hospitalisation rates of psychiatric patients (in DEXHELPP, funded by CEPHOS-Link FP7) and (iii) optimising locomotive scheduling (for ÖBB Rail Cargo Group Austria).
When it became evident that COVID-19 was going to be a global problem, the flexible nature of GEPOC meant that our research team could adapt an existing module for influenza (the mechanics of which seemed acceptably similar to COVID-19) for this new disease [L3].
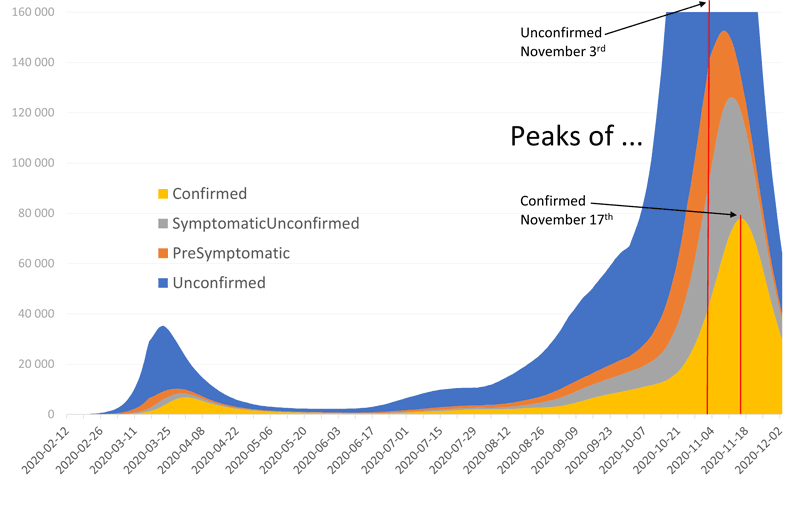
Figure 1: Daily testing of suspected COVID-19 cases yields only very limited information about the dynamic of the epidemic. But via calibration of the model it becomes possible to analyse the epidemic dynamics in detail. Our forecast on 1 November predicted the peak of confirmed cases (yellow) to take place on 17 November, and the peak of the much larger population of unconfirmed cases (blue) to take place almost two weeks earlier on 3 November. At this time the number of pre-symptomatic (orange) or still unconfirmed cases (grey), is still building up. Understanding these dynamics between subpopulations is important for timely reactions and appropriate countermeasures.
Naturally, in the early stages of the pandemic, comprehensive knowledge about the disease was not available. Nevertheless, the simulations gave highly accurate predictions, which quickly improved with increasing knowledge. The model proved useful for tasks such as: evaluating testing and tracing strategies; determining the effectiveness of different measures (e.g. how much contact reduction is necessary to bend the curve?); and calculating numbers of undetected [2] and pre-symptomatic cases (Figure 1). The research team thus became part of the advisory board for the Austrian Ministry of Health, and the Viennese and the Lower Austrian associations of hospitals commissioned dwh GmbH to simulate weekly short-term forecasts of required ICU capacities.
With the second lockdown just proclaimed by the Austrian government, the next steps will be to evaluate policies to maintain infection at a low level afterwards and to study the concurrent effect of COVID-19 and the influenza season.
Links:
[L1] http://dexhelpp.at/
[L2] https://www.dwh.at/en/blog/ministry-publishes-our-research-results-poor-measels-vaccination/
[L3] https://www.dwh.at/en/projects/covid-19/Covid19_Model_v20200929.pdf
References:
[1] M. Bicher, et al.: “GEPOC ABM: A generic agent-based population model for Austria”, in WinterSim 2018. doi: 10.1109/WSC.2018.8632170.
[2] C. Rippinger, et al.: “Evaluation of Undetected Cases During the COVID-19 Epidemic in Austria”, in Lancet Preprints, 2020. doi: 10.2139/ssrn.3689596
Please contact:
Štefan Emrich, dwh GmbH, Vienna, Austria
+43 1 256 5 256,










